The activities are as follows:
- Teacher Guide
- Student activity, Graph Type A, Level 3
- Student activity, Graph Type B, Level 3
- Student activity, Graph Type C, Level 3
- Grading Rubric
Soil is an important part of the carbon cycle because it traps carbon, keeping it out of the atmosphere and locked underground. At a global level, the amount of carbon stored by soil is more than is found in all of the plants and the atmosphere combined. Carbon trapped underground does not contribute to the rising carbon dioxide concentration in our atmosphere that leads to climate change. For decades, scientists have been researching how much carbon our soils can store to understand its role in slowing the pace of climate change.
Carbon enters the soil when plants and animals die, and their organic matter is decomposed by soil bacteria and fungi. Sometimes it is broken down into very small molecules. These molecules become attached to minerals in the soil, like clay particles. We call this mineral-associated organic matter (MAOM). The carbon is connected to minerals with very strong chemical bonds. Because these bonds are hard to break, the carbon stays in the soil for long periods of time and accumulates on clay minerals.
Some studies have shown that the carbon in MAOM can be trapped in soils for thousands of years! When more of the carbon in the soil is attached to minerals and locked in the soil for longer time periods, the ecosystem is serving an important role in keeping carbon out of the atmosphere.

Ashley is working to understand how much stable carbon there is in soils, and the role of climate. Microbes work faster in warmer and wetter conditions, which results in quicker decomposition. Ashley thought this rapid decomposition would cause organic matter to be broken down into smaller particles sooner. Therefore, she thought that in warmer or wetter environments, more soil carbon would attach to minerals and become stable MAOM. In colder or drier environments, she expected this process to happen more slowly, leading to a smaller amount of MAOM in the soil.
To test these ideas, Ashley used soil samples from forests with different climates throughout the Eastern United States. Soil samples were collected from New Hampshire to Alabama by teams of researchers using the same sampling protocol. The samples were mailed to Ashley’s lab at Indiana University for analysis. Ashley measured the amount of MAOM in each soil sample by taking advantage of a key feature: MOAM is heavy! Ashley submerged each soil sample in a saltwater solution, and the parts that floated were discarded, while the parts that sunk to the bottom were classified as MAOM. She then rinsed the salt off and measured the amount of carbon in the MAOM with an instrument called an elemental analyzer. She compared this number to the amount of carbon in the whole soil sample to calculate what percentage of the total soil carbon was attached to minerals.
Featured scientist: Ashley Lang from Indiana University
Flesch–Kincaid Reading Grade Level = 10.8
Additional teacher resources related to this Data Nugget:
- Clay content in soil can be estimated with a relatively low-tech method based on the settling time of the different soil particle sizes. For a lesson plan on this activity, click here.








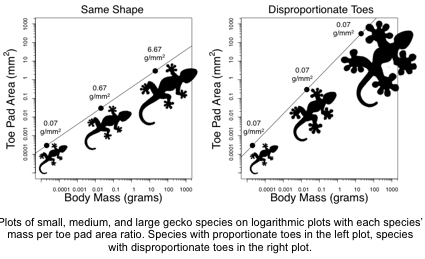
 About Travis: Ever since Travis was a kid, he was interested in animals and wanted to be a paleontologist. He even had many dinosaur names memorized to back it up! In college he discovered evolutionary biology, which drove him to apply for graduate school and become a scientist. There, he fell in love with comparative biomechanics, which combines evolutionary biology and mechanical engineering. Today Travis studies geckos and their sticky toes that allow them to scale surfaces like glass windows and tree branches.
About Travis: Ever since Travis was a kid, he was interested in animals and wanted to be a paleontologist. He even had many dinosaur names memorized to back it up! In college he discovered evolutionary biology, which drove him to apply for graduate school and become a scientist. There, he fell in love with comparative biomechanics, which combines evolutionary biology and mechanical engineering. Today Travis studies geckos and their sticky toes that allow them to scale surfaces like glass windows and tree branches.